Monogram Biosciences
True Phenotyping and Genotyping on One Report from One Sample
The addition of phenotypic to genotypic testing, as found in PhenoSense GT®Plus Integrase, is noted in US Department of Health and Human Services (DHHS) guidelines as generally preferred for patients who may benefit from drug resistance testing due to known or suspected complex drug resistance patterns.1 Monogram offers two assays that combine phenotypic and genotypic drug susceptibility data into one report:
| Drug classes tested | NRTIs, NNRTIs, PIs | NRTIs, NNRTIs, PIs, and INIs |
| Monogram test code | V7000 | M7000 |
| LabCorp test number | 551690 | 551920 |
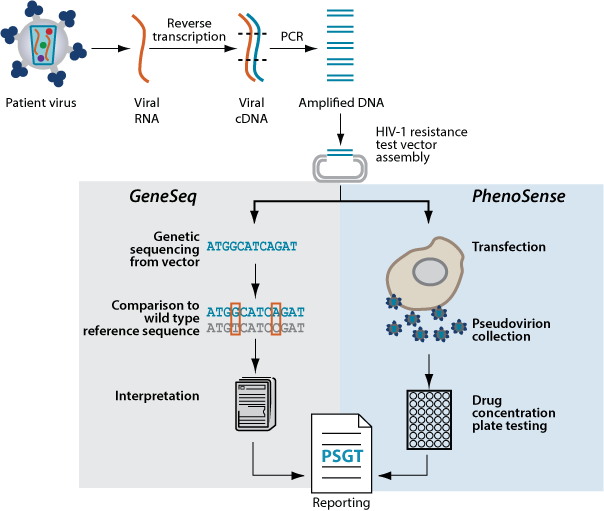
Combined Testing Process
These comprehensive tests allow advanced, practical insight into the resistance continuum and overcome limitations associated with either genotyping or phenotyping alone. Even in complex cases, PhenoSense® GT and PhenoSense GT Plus Integrase perform the genotype and phenotype from a single blood sample so that it is possible to resolve disagreement between them.