Monogram Biosciences
Genotyping vs. Phenotyping
Drug resistance testing allows clinicians to evaluate a patient’s viral resistance profile with confidence by showing which drugs are suitable to use for treatment and which drugs should be avoided. Monogram provides both genotyping (under our GenoSure® brand) and phenotyping (under our PhenoSense® brand), as well as a combination pheno/geno on a single report.
Genotyping vs. Phenotyping: What’s the Difference?
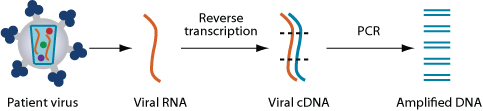
Both genotyping and phenotyping technology start out in much the same way. The patient’s viral RNA is isolated, and the section to be tested is excised, converted to DNA by reverse transcription, and then amplified via PCR. What happens after amplification, however, is very different.
Genotyping:
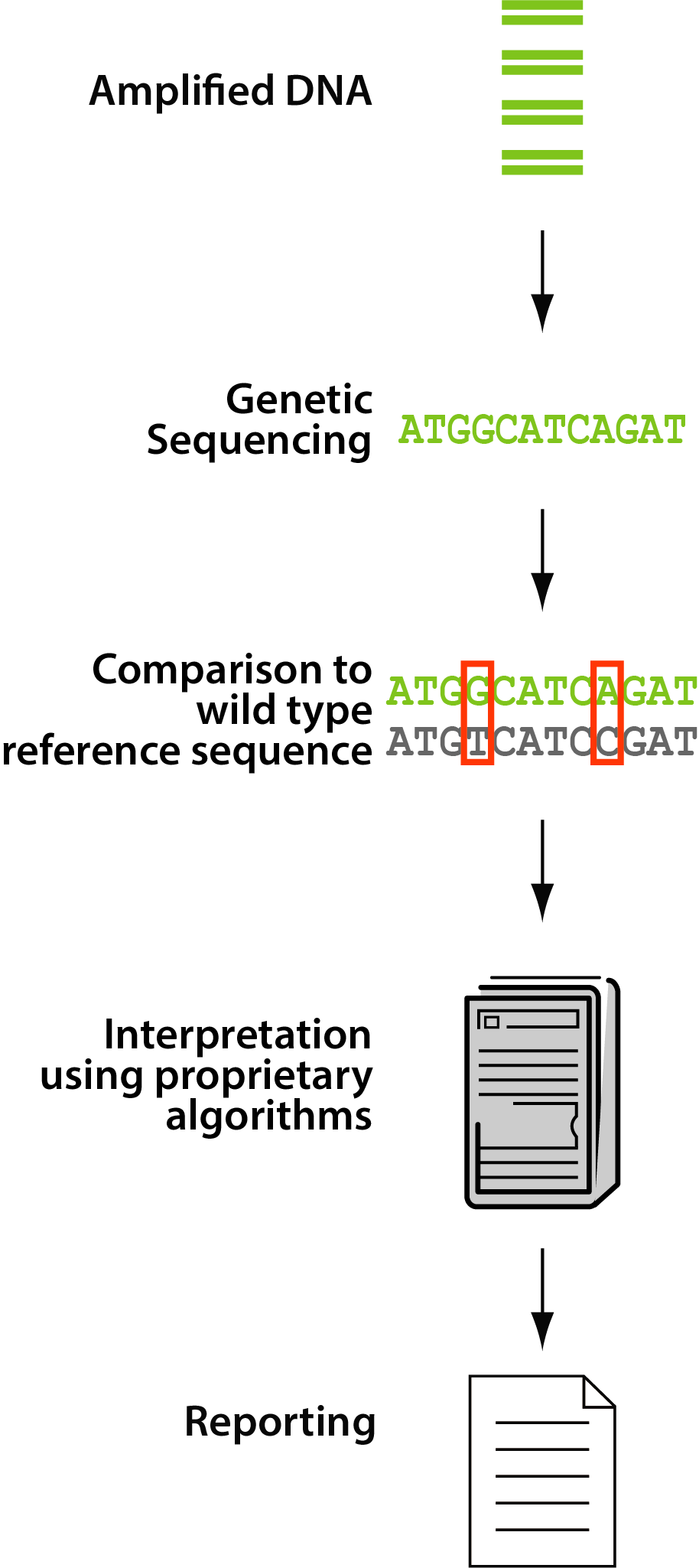
For a genotype, the amplified patient viral DNA is sequenced, compared to a wild-type reference sequence, then a prediction of drug susceptibility is made based on differences noted during the comparison. Thus, genotyping technology provides a prediction of drug susceptibility.

Phenotyping:
For a phenotype, the amplified patient-derived viral DNA segments are inserted into vector constructs, pseudovirions are created by transfecting cells with the HIV vector constructs, and then measurements of how effectively these new patient-based hybrid viruses replicate in the presence of drug are taken. Thus, phenotyping is a direct observation of how well the drugs inhibit virus replication.
Combination Phenotypic and Genotypic Testing

Genotyping and phenotyping technologies provide complementary information. Each type of technology provides a different perspective and different data about the state of a patient’s viral resistance. This is why Monogram offers the PhenoSense GT® combination test—to give clinicians the greatest amount of information about a patient’s virus. The genotyping portion provides information about key mutations, including mixtures, and the phenotyping provides in-the-moment observations of viral susceptibility to the drugs.

