Clonal Analysis
In Monogram Biosciences’ PhenoSense HIV and HCV assays, patient-derived sequences are tested as pools or libraries of sequences that capture the diversity of the viral quasi-species within a patient sample. In some instances, it may be beneficial to characterize these quasi-species to determine minor populations or changes in the viral population in the patient over time (longitudinal samples). This can be achieved using Monogram’s clonal testing assay. Patient pools generated in Monogram Biosciences’ PhenoSense HIV assays are used for selecting and growing individual clones (viruses). Typically, 48 or 96 clones (viruses) are prepared per patient sample, and they undergo an infectivity screen to determine clone viability. From these results, the client may select the clones (usually 12-16) that Monogram will use for subsequent testing, such as genotyping, drug susceptibility, and/or tropism.


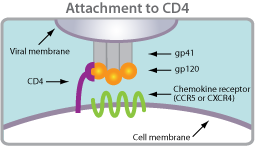
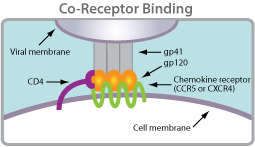
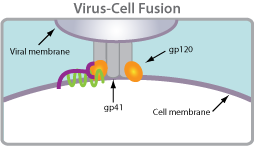
 Coreceptor tropism is defined as the ability of a particular HIV-1 virus to infect a target cell using a specific coreceptor. HIV requires two binding events to enter into a cell. It must first bind CD4 and then, secondly, a chemokine receptor. Tropism is a label given to the virus that describes which chemokine receptor the virus is using.
Coreceptor tropism is defined as the ability of a particular HIV-1 virus to infect a target cell using a specific coreceptor. HIV requires two binding events to enter into a cell. It must first bind CD4 and then, secondly, a chemokine receptor. Tropism is a label given to the virus that describes which chemokine receptor the virus is using. R5-tropic: Viruses or virus populations that can use only the CCR5 chemokine coreceptor to infect CD4+ cells.
R5-tropic: Viruses or virus populations that can use only the CCR5 chemokine coreceptor to infect CD4+ cells. X4-tropic: Viruses or virus populations that can use only the CXCR4 chemokine coreceptor to infect CD4+ cells.
X4-tropic: Viruses or virus populations that can use only the CXCR4 chemokine coreceptor to infect CD4+ cells. Dual (D)-tropic: Viruses or virus populations that can use either the CCR5 or CXCR4 coreceptors to infect CD4+ cells.
Dual (D)-tropic: Viruses or virus populations that can use either the CCR5 or CXCR4 coreceptors to infect CD4+ cells.
