- Home
- Resources
- Phenotyping
- PhenoSense Integrase
Monogram Biosciences

PhenoSense® Integrase
PhenoSense® Integrase is a commercially available phenotypic resistance assay to measure susceptibility to integrase inhibitors.
PhenoSense Integrase is used to characterize dolutegravir-, raltegravir-, and elvitegravir-resistant viruses in patients with treatment failures.
PhenoSense Integrase can be used to identify transmitted drug resistant viruses to integrase inhibitors.
Features and Benefits of PhenoSense Integrase
- Validated to be accurate, precise, sensitive, reproducible, and robust in the measurement of susceptibility to integrase inhibitors.
- Used in clinical studies of raltegravir and elvitegravir to identify and characterize raltegravir-resistant viruses in patients with treatment failure.
- Based on Monogram’s proven PhenoSense platform technology and dedication to understanding HIV-1 drug resistance.
- Along with PhenoSense GT®, provides the most complete picture of resistance to antiretroviral medications.
The Technical Process: Step by Step
1. Isolating the viral RNA
- Integrase (IN) sequences of the patient’s HIV are isolated from the patient's blood sample and amplified via reverse transcription/polymerase chain reaction (RT-PCR).
- The process is optimized and validated to capture sequences representing the viral diversity present in the patient sample.
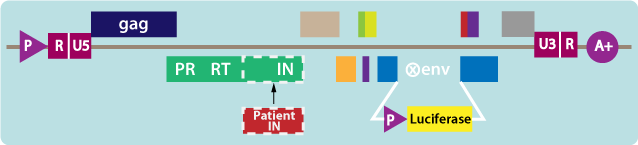
2. Constructing the test vector
- Patient HIV IN sequences are inserted into the test vector (see figure below).
- Luciferase, a light-emitting reporter gene, is present in the test vector.
3. Producing and testing the virus
- The test vector is introduced into host cells to produce virus particles that incorporate the IN fragment from the patient’s HIV.
- These virus particles are dependent on the activity of the patient’s virus IN to replicate and are used to infect target cells in the presence and absence of specific antiretroviral drugs.
- The presence of luciferase glow in the target cells indicates the ability of the virus to grow at increasing levels of drug concentration. The concentration of drug that can inhibit the growth of the virus is a measure of the susceptibility of the virus to the different drugs.
4. Measuring resistance
- The drug susceptibility of the patient-derived test virus is compared to that of a reference virus that is susceptible to all of the drugs tested.
- Susceptibility of patient virus is expressed as the fold change†, and the fold change is compared to cutoff values determined through correlation of drug susceptibility and clinical outcomes data.

