- Home
- Resources
- Suppression Management
- GenoSure Archive
Monogram Biosciences

GenoSure Archive®
GenoSure Archive® is the newest suppression management offering by Monogram Biosciences. GenoSure Archive is designed to provide HIV-1 antiretroviral (ARV) drug resistance data when standard resistance testing cannot be performed due to inadequate plasma viral load. The assay interrogates the viral archive using next-generation sequencing (NGS) methods to provide a list of the archived mutations and then assigns susceptibility calls of "sensitive", "resistant", or "resistance possible" based on those mutations.
GenoSure Archive provides valuable information for nucleoside reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), integrase inhibitors (INIs) and protease inhibitors (PIs) when considering regimen switches in virologically suppressed patients due to:
- Side effects
- Adverse events
- Regimen simplification
- Drug-drug interactions
- Concern for long-term toxicities
- Regimen intolerance
GenoSure Archive may also be considered for patients initiating therapy when conventional HIV RNA drug resistance testing is unsuccessful or unavailable as noted in the DHHS guidelines.
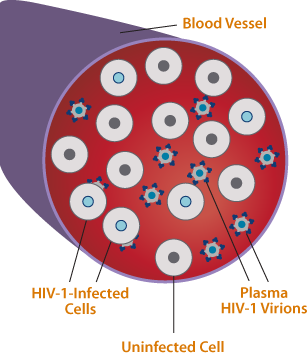
The Viral Archive—A Second Source of Resistance Information
Differences can exist between the viral population circulating in the plasma and the proviral DNA archived in infected cells.
- Viral loads and standard resistance assays analyze viral RNA in plasma. GenoSure Archive analyzes archived HIV-1 proviral DNA embedded in host cells during virus replication.
- GenoSure Archive is performed by amplifying cell-associated HIV-1 DNA from infected cells in whole blood samples then employing NGS technology to analyze the HIV-1 polymerase region, including the full-length protease and integrase coding regions and amino acids 1-400 of reverse transcriptase.
References
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/drug-resistance-testing?view=full. Accessed December 22, 2020.
De La Cruz J, Vardhanbhuti S, Sahoo M, et al. Persistence of Human Immunodeficiency Virus-1 Drug Resistance Mutations in Proviral Deoxyribonucleic Acid After Virologic Failure of Efavirenz-Containing Antiretroviral Regimens. Open Forum Infectious Diseases. 2019;6(3):1-8
Porter D, Toma J, Tan Y, et al. Clinical Outcomes of Virologically-Suppressed Patients with Pre-existing HIV-1 Drug Resistance Mutations Switching to Rilpivirine/Emtricitabine/Tenofovir Disoproxil Fumarate in the SPIRIT Study. HIV Clin Trials. 2016 Feb;17(1):29-37
HK Singh, S Jones, C Vaamonde, T Wilkin. Application of GenoSure Archive ® in Clinical Practice. Open Forum Infectious Diseases. 2016;3 (Suppl 1):1507. ID Week Abstract 1507
Mills A, Stoker A, Cai S, Petropoulos CJ, and Walworth C. HIV-1 DNA Resistance Testing Informs the Successful Switch to a Single Tablet Regimen. HIV DART and Emerging Viruses 2018. Miami, FL; November 27-29, 2018. Poster Presentation
Volpe JM, Cai S, Yang D, Petropoulos CJ, Whitcomb JM, and Walworth CM. HIV-1 DNA Resistance Testing Supports STR/2DR Treatment Simplification Options. HIV DART and Emerging Viruses 2018. Miami, FL; November 27-29, 2018. Oral Presentation
Ellis K, Nawas G, Chan C, et al. Clinical Outcomes Following the Use of Archived Proviral HIV-1 DNA Genotype to Guide Antiretroviral Therapy Adjustment. Open Forum Infectious Diseases. 2019; DOI: 10.1093/ofid/ofz533ID. Accessed March 31, 2020
Curanovic D, Martens S, Rodriguez M, et al. HIV-1 DNA Testing in Viremic Patients Demonstrates a Greater Ability to Detect Drug Resistance Compared to Plasma Virus Testing. 23rd International AIDS Conference. Virtual. July 6-10 2020
Curanovic D, Cai S, Toma J, et al. Results of repeat HIV-1 DNA Resistance Tests are Highly Concordant. ID Week 2020. Virtual Conference, October 21-25
Curanovic D, Martens S, Rodriguez M, et al. HIV-1 DNA Testing Identifies Drug Resistance in Viremic Patients With Pan-Sensitive Plasma Virus. ID Wee 2020. Virtual Conference, October 21-25

