- Home
- Services
- Clinical Trials
- HIV Testing
- PR/RT Resistance Testing
Monogram Biosciences
PR/RT Resistance Testing
Monogram has a number of tests, both phenotypic and genotypic, that focus specifically on determining resistance to drugs that target products of the PR/RT region of HIV-1 POL:
- PhenoSense GT®
- PhenoSense®
- GenoSure® MG
-
PhenoSense GT® for Reverse Transcriptase and Protease Inhibitors Assay

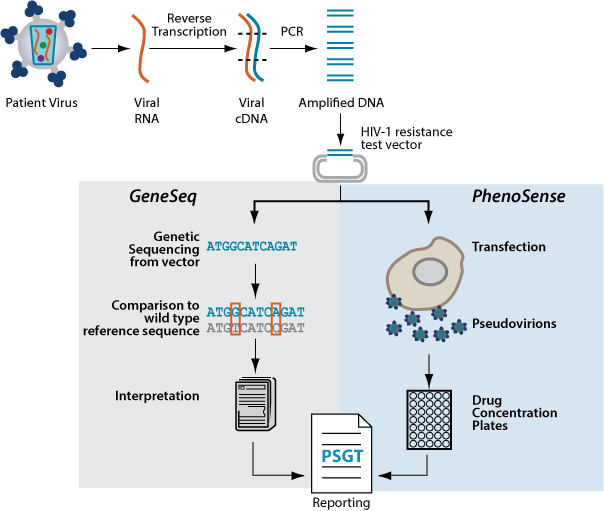
PhenoSense GT is a resistance test that combines three tests—PhenoSense® HIV, GeneSeq® HIV, and replication capacity (RC)—to make up one complete picture of drug resistance. The combination test is performed on a single blood sample, and results are reported in one report.
PhenoSense GT looks at an individual’s HIV using two different methodologies so the most effective treatment can be selected. It is a direct measure of the virus’ ability to replicate in given concentrations of antiviral compounds as measured by the phenotypic portion of PhenoSense GT. The patient's virus is also sequenced, with the genotypic data provided alongside the susceptibility results. Finally, PhenoSense GT measures the ability of the viral protease and reverse transcriptase to drive replication—known as replication capacity, one component of viral fitness.
PhenoSense GT uses advanced technology and offers consistent and clear results, because both phenotypic and genotypic results come from the same blood sample, and some of the discrepancies between phenotypic measurements and genotypic predictions are resolved as part of the assay.
The report form includes both phenotypic and genotypic drug resistance information for all of the currently approved nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).
Features and benefits of the PhenoSense GT assay include:
- Combines state-of-the-art phenotypic test results (PhenoSense HIV) with state-of-the-art genotypic test results (GeneSeq HIV).
- Provides results on a single report with side-by-side phenotypic and genotypic resistance data.
- Supplements resistance results with replication capacity information to evaluate viral fitness.
- Overcomes limitations associated with the use of a single method alone.
- Avoids complications associated with separate samples and separate laboratories by performing both tests on one patient sample.
- HIV subtype reporting.
PhenoSense GT is an assay frequently used to determine OBT to be given with investigational agents in clinical trials.
 Download Sample Report(Opens in a new window) Download PhenoSense® GT Process Slide(Opens in a new window)
Download Sample Report(Opens in a new window) Download PhenoSense® GT Process Slide(Opens in a new window)Parkin N, Chappey C, Maroldo L, Bates M, Hellmann NS, Petropoulos CJ. Phenotypic and genotypic HIV-1 drug resistance assays provide complementary information. J Acquir Immune Defic Syndr. 2002;31(2):128-136.
-
PhenoSense HIV for Reverse Transcriptase and Protease Inhibitors Assay

PhenoSense HIV is a phenotypic HIV drug resistance assay and is a widely used phenotypic test. PhenoSense HIV utilizes proprietary, leading-edge technology for a direct, rapid, and precise measure of a patient’s virus’ sensitivity to antiretroviral drugs. Test results allow health care professionals to review the level of susceptibility or resistance that an individual’s virus has to each antiretroviral drug in order to design an individualized treatment plan. PhenoSense HIV drug resistance results are also provided with one component of viral fitness, replication capacity (RC).
The report form includes drug resistance information for all of the approved nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs).
Features and benefits of the PhenoSense HIV assay:
- Provides direct measurement of drug susceptibility.
- Measures quantitative susceptibility data—can measure partial susceptibility and hypersusceptibility.
- Uses susceptibility cutoffs to determine when the likelihood of response begins to decrease.
- Includes a logical, easy-to-read report form.
- Gives accurate results using plasma samples with viral loads as low as 500 HIV RNA copies/mL.
- Supplements resistance results with replication capacity information to evaluate viral fitness.
 Download Sample Report(Opens in a new window) Slide Presentation Featuring Vector and Transfection Schematics(Opens in a new window)
Download Sample Report(Opens in a new window) Slide Presentation Featuring Vector and Transfection Schematics(Opens in a new window)Reference
Petropoulos C, Parkin NT, Limoli KL, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44(4):920-928. -
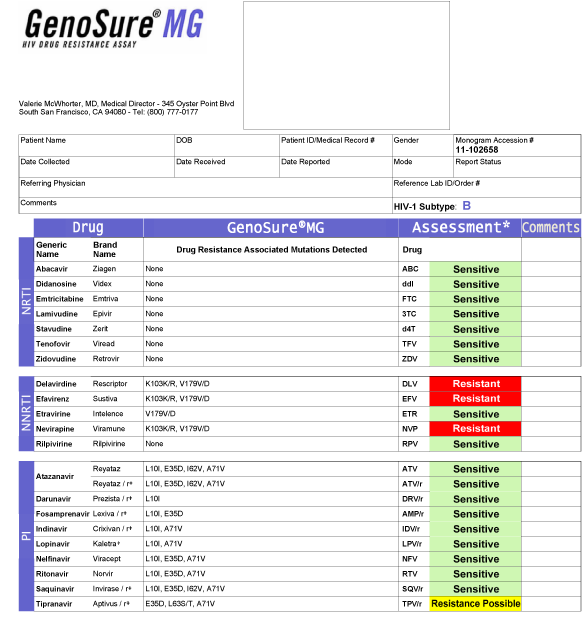
GenoSure® MG

GenoSure MG is a state-of-the-art HIV genotyping assay with laboratory processes that allow for a rapid turnaround time combined with resistance predictions based on Monogram’s proprietary genotypic assessment. This assessment is unique and is frequently updated through detailed analysis of Monogram’s vast genotypic/phenotypic database, as well as literature review and joint analyses of clinical trials data with our academic and pharmaceutical company collaborators.
The report form includes drug resistance information for all of the approved nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs). In addition, all changes observed relative to reference are reported for all of protease and amino acids 1-400 of reverse transcriptase.
Features and benefits of the GenoSure assay:
- A highly accurate algorithm.
- Rapid turnaround time (7-9 days).
- Over 99% reproducibility.
- Can be reliably performed on samples with viral loads as low as 500 copies/mL.
- Sensitivity to detect mixtures as low as 10% of the total viral population.
- Includes HIV-1 subtype reporting.