Monogram Biosciences
HIV Phenotypic Testing
Phenotypic testing is one of two types of HIV drug resistance testing. Phenotypic testing is performed by exposing a sample of an individual’s HIV to all of the available antiretroviral drugs.
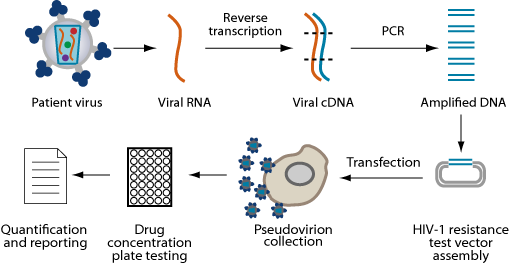
By directly measuring the ability of HIV to grow in the presence of these drugs, scientists can determine which drugs are suitable for treatment and which drugs should be avoided. The activity of a patient’s HIV in the presence of the antiretroviral drugs is compared to the activity of a control strain of HIV that is known to be susceptible to a specific drug. This comparison can determine how likely or unlikely a person is to respond to that drug. The results of phenotypic testing can be used by a health care team to select the most active antiretroviral regimen for a particular individual.
Replication Capacity
Replication capacity measures how well a patient’s virus is able to replicate compared to a wild-type reference virus in the absence of drug. Although drug resistance mutations enable viruses to replicate in the presence of a drug, they may do so at some fitness cost to the viruses. Replication capacity is related to, but not equivalent to, viral fitness, which takes into account viral and immunologic pressure. Understanding how fit a patient’s virus is may provide insight in a highly complex case. Replication capacity is included with PhenoSense® GT, PhenoSense®, and PhenoSense® Integrase.

