- Home
- Services
- Clinical Trials
- HIV Testing
- Integrase Resistance Testing
Monogram Biosciences
Integrase Resistance Testing
Integrase inhibitors (INIs) are a potent class of antiretrovirals that promote rapid virologic declines in patients by targeting the activity of HIV-1's integrase enzyme. Monogram has adapted its resistance testing technology to provide both phenotypic and genotypic susceptibility information for new integrase inhibitors under development. Both of Monogram’s integrase assays, PhenoSense® Integrase and GeneSeq® Integrase, are actively used in ongoing clinical studies of new integrase inhibitors.

GeneSeq® HIV Integrase
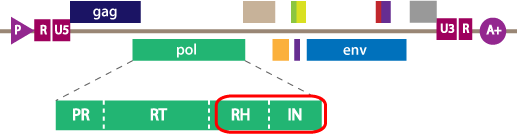
The GeneSeq HIV Integrase assay provides complementary sequence data of integrase and RNaseH. We apply Monogram’s expertise in viral sequencing to the RH and IN portions of HIV-1 pol, reporting out polymorphisms and resistance-associated mutations. With integrase inhibitors (INIs) available in the clinic and in development, understanding the mutations that confer resistance is essential for understanding the nature and complexity of viral resistance.

PhenoSense® RH/IN
Monogram Biosciences’ PhenoSense RH/IN assay is a novel phenotypic assay that measures the susceptibility of inhibitors of HIV-1 integrase (IN) and RNaseH (RH), as well as the replication capacity of patient viruses with reduced susceptibility to such inhibitors. With integrase inhibitors (INIs) available in the clinic and in development, there is considerable need for a phenotypic assay that monitors drug susceptibility in patients, as well as assisting with antiviral drug discovery and development. Monogram’s PhenoSense HIV assay has been adapted to measure inhibition of recombinant viruses containing patient-derived integrase coding sequences by INIs.

